What are Polycyclic
Aromatic Hydrocarbons?
Polycyclic Aromatic Hydrocarbons (PAHs) are a group of chemicals
that occur naturally in coal, crude oil and gasoline. However they become
pervasive mainly as a by-product of incomplete combustion. The less efficient
the burning process, the more PAHs is given off. Forest fires and volcanoes can
produce PAHs naturally, however in the urban context; the most common sources
of PAHs are tobacco smoke, automobile exhaust, grilled or charbroiled meat.
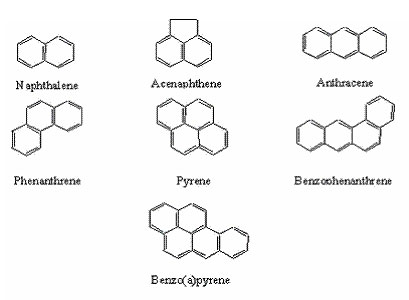
Although hundreds of PAHs exist, two of the more common ones are
benzo(a)pyrene and naphthalene.
How can I be exposed to
PAHs?
PAHs are found throughout the environment in the air, water and
soil, and can remain in the environment for months or years. Levels of PAHs in
urban air may be 10 times greater than those found in rural areas. You also may
be exposed to PAHs in soil near hazardous waste sites or near areas where coal,
wood, gasoline or other products have been burned.
In the home, PAHs are present in tobacco smoke, smoke from wood
burning stoves and fireplaces, creosote-treated wood products and some foods.
Barbecuing, smoking or charring food over a fire greatly increases the amount
of PAHs in the food.
Other foods that may contain low levels of PAHs include roasted
coffee, roasted peanuts, refined vegetable oil, grains, vegetables and fruits.
A variety of cosmetics and
shampoos are made with coal tar and therefore may contain PAHs.
The PAH compound naphthalene is present in some mothballs and
cleaners.
How can PAHs affect my
health?
The health effects that can be caused by exposure to PAHs depend
on:
- how much has entered your body,
- how long you have been exposed
to PAHs and
- how your body responds to PAHs.
It is not clear whether PAHs cause short-term health effects.
Other chemicals commonly found with PAHs may be the cause of short-term
symptoms such as eye irritation, nausea, vomiting, diarrhea and confusion.
Possible long-term health effects caused by exposure to PAHs may
include cataracts, kidney and liver damage and jaundice. Repeated skin contact
with the PAH naphthalene (found in some mothballs) can result in skin redness
and irritation. Breathing or swallowing large amounts of naphthalene can cause
the breakdown of red blood cells.
Some people who have breathed or touched mixtures of PAHs and
other chemicals for long periods of time have developed cancer. Some PAHs have
caused cancer in laboratory animals when they breathed air containing them,
ingested them in food or had them applied to their skin.
PAHs are stored in the
fat tissue of the breast and can increase the propensity for breast cancer.
When PAHs are associated with aryl hydrocarbon receptor (AhR), a protein, a
series of cellular changes is initiated leading to altered cell signals that
increase DNA mutation. PAHs can also be genotoxic, as they are able to interact
with the genes directly causing DNA damages.